Since PDH needs R(+)-Lipoic Acid as a coenzyme for its function, you might expect that giving cells extra R(+)-Lipoic Acid would make PDH extract even more energy from pyruvic acid. Not so: when a team of scientists provided cultured cells with R(+)-lipoic acid, it was found to have no effect on PDH's ability to help process pyruvic acid 67. That might seem strange, but it actually does make sense, since the cell normally has all the R(+)-Lipoic Acid it needs for PDH activity. It's precisely because it has no need for extra lipoic acid that, after "charging up" supplemental R(+)-Lipoic Acid into its more potent DHLA form, PDH readily releases the extra DHLA into the cell and the rest of the body, where it can lend its potent antioxidant assistance.
But when the same scientists provided the cells with R(+)-lipoic acid's "evil twin," they found that S(-)-lipoic acid actually suppresses the ability of cells to use pyruvate in energy production, reducing its activity by 25 to 30%! The obvious explanation: S(-)-lipoic acid was actually getting in the way of the natural R(+)-form of lipoic acid, which is needed for PDH to do its job. Looking at their results, the scientists concluded that "R(+)-LA [that is, R(+)-lipoic acid], and not a racemic mixture of R(+)- and S(-)-LA, should be considered a choice for therapeutic applications." 67
But hold on. If R(+)-Lipoic Acid doesn't increase PDH activity, doesn't this mean that R(+)-Lipoic Acid is useless for boosting mitochondrial energy production? No - because the effects of R(+)-Lipoic Acid on mitochondrial energy production go well beyond PDH, and into the very heart of the activity of the mitochondria's ATP turbine.
This was first shown by German scientists, in early experiments using mitochondria which had literally been turned inside out to study their functioning. 11 These researchers found that providing these special mitochondria with R(+)-Lipoic Acid boosts their ATP production. But they also found that the two forms of lipoic acid showed "decisive differences" in their effects on mitochondrial ATP production. Exposing these mitochondria to either the S(-)- form or the racemate actually slows their rate of ATP synthesis!
Why would R(+)-Lipoic Acid tubocharge mitochondrial function, while the S(-)-enantiomer undermines it? Remember that the "leakiness" of the mitochondrial membrane is in large part caused by damage to the proteins it contains. One critical kind of damage that these proteins undergo is the handcuffing together (crosslinking) of key proteins' sulfur-rich cysteine amino acids, which creates disulfide (sulfur-to-sulfur) crosslinks between two sulfur atoms. These disulfide handcuffs change the structure of the membrane itself and of its functional proteins - including the all-important Complex V turbines. It's these structural changes that create the "holes" in the "dam" of the mitochondrial inner membrane, rendering the mitochondrion an inefficient and "dirty" energy producer.
Preventing the crosslinking of sulfur groups is therefore a key part of keeping the mitochondrial inner membrane intact, and its proteins functional. But in order to maintain the structure and function of this system, a molecule must be able to interact with the complex 3-dimensional structures of the proteins themselves. So it's easy to see why scientists studying the effects of the two forms of lipoic acid have concluded that the specific three-dimensional design of R(+)-Lipoic Acid allows it to favorably interact with mitochondrial proteins - including the turbines of Complex V - while the mirror-opposite architechture of the S(-)-form cannot.
In other words, you won't get anywhere trying to install a turbo charger in your car if it isn't compatible with your engine. Trying to install the wrong unit might even sap your engine's power.
Learn More About Energy in Crisis
Please consult with a health care professional before starting any supplementation program. The information contained on this site is general in nature and Company does not take any responsibility for any errors that may appear. Company has made every attempt to make the information as accurate as possible. However, Company does not warrant its accuracy. Please note that the statements on this web site have not been evaluated by the FDA.




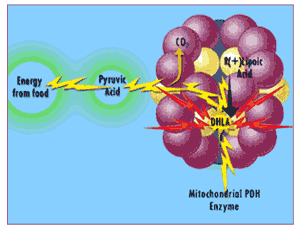 So you'd expect that boosting levels of R(+)-Lipoic Acid might increase the mitochondria's ability to make ATP. (And you'd be right - but, as we'll see, for the wrong reason). And because the S(-)-form is only poorly used as an energy coenzyme by the mitochondria - and, indeed, can actually interfere with the mitochondria's ability to use R(+)-Lipoic Acid for this purpose! - it also makes sense to look and see if the two lipoic acid enantiomers might have different effects on energy production and use.
So you'd expect that boosting levels of R(+)-Lipoic Acid might increase the mitochondria's ability to make ATP. (And you'd be right - but, as we'll see, for the wrong reason). And because the S(-)-form is only poorly used as an energy coenzyme by the mitochondria - and, indeed, can actually interfere with the mitochondria's ability to use R(+)-Lipoic Acid for this purpose! - it also makes sense to look and see if the two lipoic acid enantiomers might have different effects on energy production and use.


