Antioxidant Antagonist
An Antioxidant...and its Antagonist
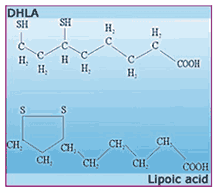
Despite what you hear, lipoic acid itself - whether we're talking about the natural R(+)-enantiomer or the artificial S(-)-form - is not much of an antioxidant. The real free-radical fighter in the lipoic acid story is dihydrolipoic acid
30 Lipoic acid and DHLA are like the two alter egos of some superheroes. If lipoic acid is Billy Batson, then DHLA is Captain Marvel. One (lipoic acid) is the day-to-day identity of the hero: courageous and smart, but limited to the strengths of a mere mortal. But when the hero puts on a ring or says a magic word, the day-to-day identity is shed in a flash of light, as the hero is charged with fabulous powers.
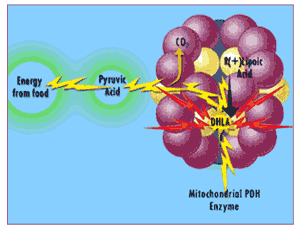
 Lipoic acid is "charged up" into its DHLA "superhero" identity as part of its function in transforming food energy into cellular energy in the mitochondrial enzyme complex pyruvate dehydrogenase.
Lipoic acid is "charged up" into its DHLA "superhero" identity as part of its function in transforming food energy into cellular energy in the mitochondrial enzyme complex pyruvate dehydrogenase.
"R(+)-lipoic acid is "charged up" into DHLA as part of its role in energy metabolism"
Usually, mitochondria have to synthesize their own lipoic acid 31 which is an "expensive" process … so there's just enough lipoic acid available to meet the mitochondria's energy needs. When you make extra lipoic acid available by taking a supplement, the mitochondria "charge up" lipoic acid into DHLA, whereupon the extra DHLA is released into the rest of the cell and into the surrounding fluid... 32 and free radicals tremble in their boots.
So what are the "superpowers" that make DHLA the real hero of our tale? DHLA is both a more potent antioxidant than lipoic acid itself (whether as R(+)-or S(-)-), and has a wider range of antioxidant actions. 30 For instance, one key property of lipoic acid that we've already mentioned is its ability to "recharge" the complete antioxidant network, giving new life to molecules of vitamin C, E- complex vitamins, and glutathione, 30 as well as CoQ10, 33 when they fall down in the fight against free radicals. But it's actually DHLA, and not lipoic acid itself, that has this ability. 30 Likewise, most studies find that DHLA can take out superoxide, the main free radical made by the cell's mitochondria 34 and DHLA appears to even prevent the formation of superoxide by the mitochondria 35 Lipoic acid itself lacks this ability. And most remarkably, whereas conventional antioxidants just prevent free radical damage, DHLA is actually able to help repair free-radical damage to some types of bodily proteins, by enhancing the activity of a protein repair enzyme 36
 What difference does this make to the form of lipoic acid you choose? Simple. Remember, R(+)-Lipoic Acid is the form of the molecule actually used by your body. R(+)-lipoic acid, and not the S(-)-form is made by your mitochondria, and is essential to their function. So it's no surprise that the mitochondrial enzyme complex (PDH) which is specifically responsible for converting lipoic acid into DHLA "prefers" the orthomolecular R(+)-enantiomer to the foreign S(-)-form: R(+)-Lipoic Acid is the "key" made by your body to open this "lock," while the S(-)-form is a badly-made copy.
What difference does this make to the form of lipoic acid you choose? Simple. Remember, R(+)-Lipoic Acid is the form of the molecule actually used by your body. R(+)-lipoic acid, and not the S(-)-form is made by your mitochondria, and is essential to their function. So it's no surprise that the mitochondrial enzyme complex (PDH) which is specifically responsible for converting lipoic acid into DHLA "prefers" the orthomolecular R(+)-enantiomer to the foreign S(-)-form: R(+)-Lipoic Acid is the "key" made by your body to open this "lock," while the S(-)-form is a badly-made copy.
In fact, the mitochondrial PDH enzyme complex converts R(+)-Lipoic Acid into DHLA at a rate at least twenty-four times faster than the S(-)-form. 37 38 , In some human cell types, the mitochondrial enzyme won't accept S(-)-lipoic acid at all. 38 Worse: at high concentrations the S(-)-enantiomer actually interferes with the mitochondrial enzyme's ability to make DHLA from R(+)-lipoic acid! 37 Fortunately, it's unlikely that anyone taking racemate lipoic acid supplements is in danger of getting such high concentrations of the S(-)-enantiomer into their bodies.
There are, however, places other than the mitochondria where the body can make some DHLA from either form of lipoic acid. As a result, when you look at the total DHLA formed in the cell, as opposed to just what's made in the PDH complex, the S(-)-enantiomer is still clearly inferior to the R(+)-, but the gulf is not quite so extreme: in the heart, for instance, R(+)-Lipoic Acid is "only" transformed into DHLA six to eight times more quickly than is the S(-)-form. 39
Even this, however, makes the S(-)-form look more useful than it really is, because the main way that the S(-)-form gets powered up into DHLA is by hijacking the activity of an enzyme which was never designed for the purpose: glutathione reductase. You may know glutathione (GSH) as another player in the antioxidant network , which is known specifically for its ability to protect the liver against toxins and drugs and to fight lung infections. 40 Glutathione reductase is an enzyme whose purpose is to recycle used-up glutathione (GSH) into its active form.
Well, there's only so much an enzyme can do at a time! Every moment that glutathione reductase is kept sidetracked by S(-)-lipoic acid is a moment during which it can't do the job it was designed to do - namely, again, to keep glutathione cycling smoothly through the cell's defense system. So when S(-)-lipoic acid takes over this enzyme, a bit more DHLA is made … but a bit less glutathione is recycled, too. Bottom line: the S(-)-enantiomer robs Peter (GSH) to pay Paul (DHLA), giving with one hand while taking with the other. It's one step forward, one step back. R(+)-Lipoic Acid has no such problems, being strongly taken up by the mitochondrial enzyme as it was designed to do, and having a much weaker tendency to waste glutathione reductase's time.
But enough molecular babble (for now!). What does all of this mean, in terms of real-world antioxidant defense? Scientists have been asking themselves this question for some time, and have made some discoveries that users of lipoic acid need to know about. Let's have a look at their findings.
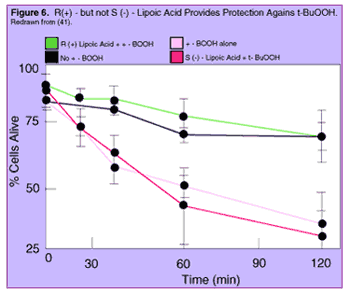
One study 41 looked at the effects of aging - and of the two forms of lipoic acid - on the vulnerability of liver cells to tert-butylhydroperoxide (t-BuOOH), a chemical that causes the cell's mitochondria to churn out more free radicals. As had been seen in other studies, older animals' cells were much more susceptible to the toxin than were those from young animals: an amount of t-BuOOH that half of the cells from young animals managed to survive, was enough to kill all but 12% of the cells from older ones.
Astoundingly, when the cells of old animals were given one of the two forms of lipoic acid in advance of t-BuOOH, R(+)-Lipoic Acid completely protected the cells from the free radical assault, so that the cells given R(+)-Lipoic Acid and the toxin survived as often as did cells which were not given the toxin at all. And, on the opposite extreme, S(-)-lipoic acid provided no significant protection against rampaging free radicals, such that cells were equally doomed by the toxin whether or not they also got the S(-)-form.





 Lipoic acid is "charged up" into its DHLA "superhero" identity as part of its function in transforming food energy into cellular energy in the mitochondrial enzyme complex pyruvate dehydrogenase.
Lipoic acid is "charged up" into its DHLA "superhero" identity as part of its function in transforming food energy into cellular energy in the mitochondrial enzyme complex pyruvate dehydrogenase. What difference does this make to the form of lipoic acid you choose? Simple. Remember, R(+)-Lipoic Acid is the form of the molecule actually used by your body. R(+)-lipoic acid, and not the S(-)-form is made by your mitochondria, and is essential to their function. So it's no surprise that the mitochondrial enzyme complex (PDH) which is specifically responsible for converting lipoic acid into DHLA "prefers" the orthomolecular R(+)-enantiomer to the foreign S(-)-form: R(+)-Lipoic Acid is the "key" made by your body to open this "lock," while the S(-)-form is a badly-made copy.
What difference does this make to the form of lipoic acid you choose? Simple. Remember, R(+)-Lipoic Acid is the form of the molecule actually used by your body. R(+)-lipoic acid, and not the S(-)-form is made by your mitochondria, and is essential to their function. So it's no surprise that the mitochondrial enzyme complex (PDH) which is specifically responsible for converting lipoic acid into DHLA "prefers" the orthomolecular R(+)-enantiomer to the foreign S(-)-form: R(+)-Lipoic Acid is the "key" made by your body to open this "lock," while the S(-)-form is a badly-made copy.


 In another study 42 nerve cells from different parts of the brain were exposed to enough buthionine sulfoxamine (BSO) to destroy half of them. (BSO is a chemical that makes cells more vulnerable to free radicals by depleting the cell of antioxidant defenses). Providing the cells with R(+)-Lipoic Acid saved between one half and one third of the brain cells that would otherwise have died from necrotic cell death (depending on what kind of brain cells were involved). By contrast, neither the S(-)-form, nor the racemate (R,S)-lipoic acid found in common supplements, offered any significant protection.
In another study 42 nerve cells from different parts of the brain were exposed to enough buthionine sulfoxamine (BSO) to destroy half of them. (BSO is a chemical that makes cells more vulnerable to free radicals by depleting the cell of antioxidant defenses). Providing the cells with R(+)-Lipoic Acid saved between one half and one third of the brain cells that would otherwise have died from necrotic cell death (depending on what kind of brain cells were involved). By contrast, neither the S(-)-form, nor the racemate (R,S)-lipoic acid found in common supplements, offered any significant protection.